
Chapter5
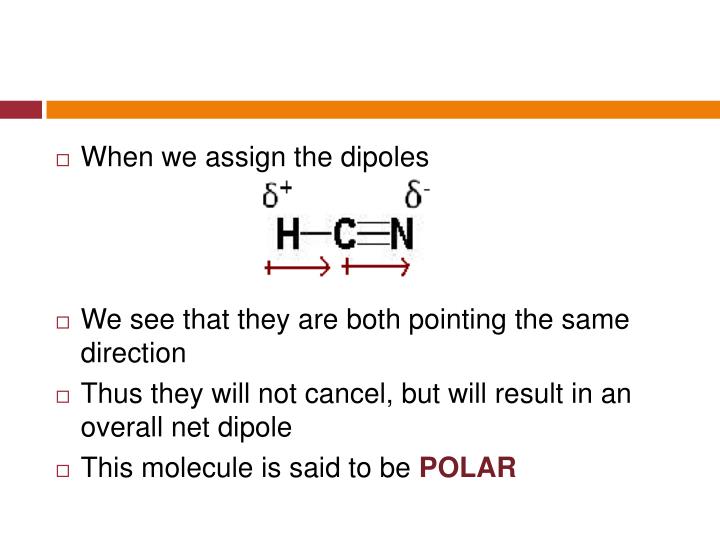
So, is HCN polar or Nonpolar? HCN is a polar molecule because of the large electronegative difference between Nitrogen (3.04) and hydrogen (2.2) due to which the linear-shaped molecule has unequal sharing of charge and results in non zero dipole moment making the molecule polar. HCN is acidic in nature.

have a question about water The Student Room
Since this species is charged, the terms "polar" and "nonpolar" are irrelevant. 5. HCN : linear. polar: Linear molecules are usually nonpolar, but in this case, not all of the atoms connected to the central atom are the same. The C—N bond is polar, and is not canceled out by the nonpolar C—H bond. 6. CO 2: linear. nonpolar: The.
PPT Polar Bonds and Molecules PowerPoint Presentation ID544532
Hydrogen Cyanide (HCN) is a polar molecule due to a large electronegativity difference between the nitrogen and hydrogen atoms across the linear molecule. The molecule is made up of two polar bonds with opposite polarities. As a result, one end of the molecule has a partial positive charge and the other end has a partial negative charge.

Hcl Polar or Nonpolar HallietaroSimmons
Is HCN polar or non-polar? Why? Polar Compounds: If a structure of a molecule is symmetrical, the overall dipole moment of the molecule will be zero, and the bond will become non-polar..

Hcn Polar Or Nonpolar soakploaty
Find the net dipole moment (you don't have to actually do calculations if you can visualize it) If the net dipole moment is zero, it is non-polar. Otherwise, it is polar. Example 4.12. 1: Label each of the following as polar or nonpolar. Water, H 2 O: Methanol, CH 3 OH: Hydrogen Cyanide, HCN: Oxygen, O 2:

Best Overview Is HCl polar or nonpolar [1] Science Education and
Learn to determine if HCN is polar or nonpolar based on the Lewis Structure and the molecular geometry (shape).We start with the Lewis Structure and then use.

PPT Polar or Nonpolar PowerPoint Presentation, free download ID3483667
Is HCN Polar or Nonpolar? (Hydrogen Cyanide) Geometry of Molecules 2.71K subscribers 859 views 1 year ago Polarity of Molecules Hey Guys ! Today in this video we are going to determine the.
Is H2 Polar Or Nonpolar Asking List
To determine if a molecule overall is polar or nonpolar you must look at both the electronegativity differences in each bond and the shape of the molecule. HCN is a linear molecule with a single bond between the H and the C and a triple bond between the C and the N. Looking at the electronegativities H Step 2: Identify each bond as either polar or nonpolar. (If the difference in electronegativity for the atoms in a bond is greater than 0.4, we consider the bond polar.. The Lewis structure and geometric sketch for HCN are the same: The electronegativities of hydrogen, carbon, and nitrogen are 2.20, 2.55, and 3.04. The 0.35 difference in. Is HCN polar or nonpolar? | Quizlet In a single graph, sketch ν = 100 cos (ωt + Φ) versus ωt for Φ = 90°, 45°, 0°, -45°, and -90°. a) State whether the voltage function is shifting to the right or left as Φ becomes more negative. b) What is the direction of shift if Φ changes from 0 to 45°? HCN, or hydrogen cyanide, is a polar molecule because there is a large electronegative difference between the N and H across the linear molecule. It consists of two polar bonds whose polarities line up in the same direction. Thus conferring an overall partial positive charge on one end of the molecule and a partial negative on the other end. [1] Hydrogen Cyanide is a colorless, flammable, and poisonous chemical liquid. Represented by the chemical formula, HCN is one of those molecules that has an interesting Lewis structure. This liquid is used in electroplating, mining, and as a precursor for several compounds. HCN Lewis Structure (Hydrogen Cyanide) Watch on 0:00 / 2:55 May 24, 2023 by Jay Rana HCN is a POLAR molecule. But why? And how can you say that HCN is a polar molecule? Want to know the reason? Let's dive into it! HCN is a POLAR molecule because the Carbon-Nitrogen bond present in the molecule is polar, which causes the partial positive (ẟ+) and partial negative (ẟ-) charge to appear on the molecule. Hydrogen cyanide (HCN) is a colorless or pale blue liquid or gas with an odor similar to that of an almond. Hydrocyanic acid, or prussic acid, is a solution of hydrogen cyanide in water. Hydrogen cyanide and its compounds are used in a wide variety of chemical processes, including fumigation, case hardening, electroplating, and ore concentration. Build the molecule HCN in the simulator based on the following Lewis structure:. Also, polar solvents are better at dissolving polar substances, and nonpolar solvents are better at dissolving nonpolar substances. Figure 7.28 (a) Molecules are always randomly distributed in the liquid state in the absence of an electric field. (b) When an. The molecular weight of HCN is 27.025 g/mol. The boiling point of the compound is 78.1 deg F and the melting point is 7.9 deg F. Below are the reactions or methods which lead to the creation of this compound: When methane reacts with ammonia and oxygen we get hydrogen cyanide and water.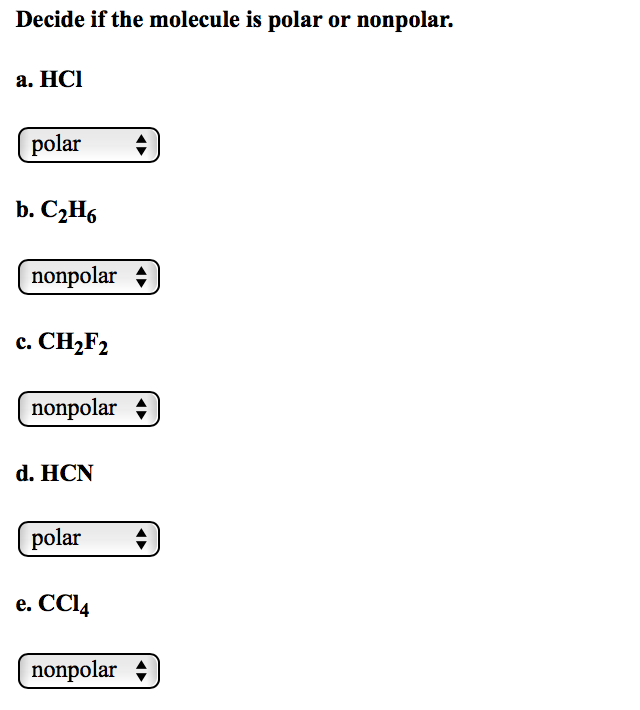
Solved Label the bond formed between I and each of the

Hcn Polar Or Nonpolar soakploaty
[Solved] image attached 1. Complete the table below. Indicate whether
How Do You Teach Polar Vs. Nonpolar Molecules? — CoScine Creative
How To Know If A Molecule Is Polar Or Nonpolar Khan Academy
Polar and Nonpolar Covalent Bonds Characteristics & Differences
Hcn Polar Or Nonpolar soakploaty

Which of the following contains both polar and nonpolar covalent bonds